- Mitral-valve prolapse is defined as the displacement of some portion of one or both leaflets of the mitral valve into the left atrium during systole.1
- Estimated to affect 2% to 3% of individuals2
- Although mitral-valve prolapse is more common in women, more men are referred for surgery.1
- The natural history of mitral-valve prolapse is heterogeneous and is largely determined by the severity of mitral regurgitation. Although a majority of patients remain asymptomatic and may have a near-normal life expectancy, approximately 5 to 10% have progression to severe mitral regurgitation.1
- Left untreated, mitral valve prolapse with severe MR results in limiting symptoms, left ventricular dysfunction, heart failure, pulmonary hypertension, and AFib.1
Anatomy
- Mitral-valve prolapse is characterized predominantly by myxomatous degeneration.1
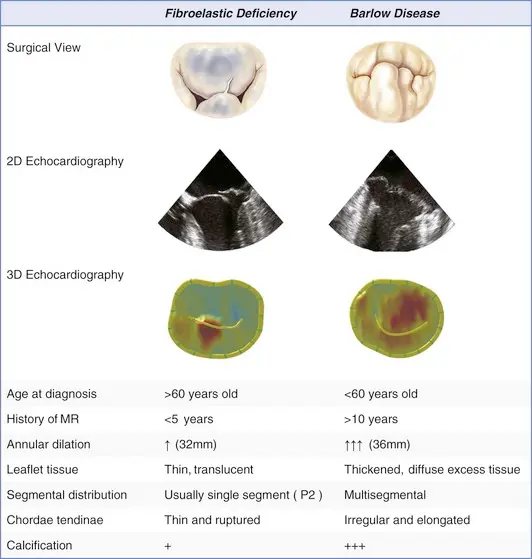
- In younger patients, the disease is often manifested by excess leaflet tissue and is known as Barlow’s syndrome, the most extreme form of myxomatous degeneration.
- On the other hand, in older patients 👵, the prolapsing mitral valve tends not to have excess leaflet tissue, an entity known as fibroelastic deficiency.
- Both conditions can lead to leaflet prolapse and chordal elongation or rupture, representing the spectrum of degenerative mitral-valve disease.
- Involves myxomatous degeneration of the mitral valve leaflets → eventually leads to structural incompetence and superior displacement of one or both mitral leaflets into the left atrium (LA) during systole 2
- Anatomic abnormalities result in the mitral orifice not closing completely during systole, causing regurgitation.1
- Annular dilatation may also develop over time, leading to further progression of mitral regurgitation.1
Barlow disease
- diffuse, myxoid degeneration of the MV → excess tissue in multiple valve segments, including leaflets and chordae → (typically) both anterior and posterior MVP, as well as annular dilation
- Compared to fibroelastic deficiency, Barlow disease is less frequently associated with chordal rupture.
Fibroelastic deficiency
- Caused by abnormalities in connective tissue → leaflet redundancy, chordal thinning and elongation → MVP
- The thinned and elongated tendinous chords in MVP are prone to rupture
- Chordal rupture may lead to flail leaflet and a sudden ↑ in MR volume (acute MR)
Diagnosis
- Physical Exam
- 🩺: mid‐systolic “click” heard best at the apex
- presence of a late systolic or holosystolic murmur can suggest the presence of MR
- 🩺: mid‐systolic “click” heard best at the apex
- Diagnosis
- Echo (gold standard)
- TTE to assess the mechanism and severity of mitral regurgitation, as well as LA, LV size and function
- ECG to assess cardiac rhythm
- Echo (gold standard)
Echo
- Using echocardiography, MVP is diagnosed ideally in the PLAX window as systolic displacement of the mitral leaflet into the LA of ≥2 mm from the mitral annular plane.
- If parasternal windows are of poor quality, the apical long-axis view can also be used, although the latter is less standardized and thus more variable.
- ⛔ Diagnosis of MVP should be avoided in the A4C or A2C windows
MR quantification in MVP
⛔ Avoid using single frame measurements - PISA EROA and VCW - when the MR is not holosystolic, e.g. MVP with late systolic MR, functional MR with biphasic MR, LV dyssynchrony with early systolic MR, as they can overestimate MR severity.
When non-holosystolic, you should use regurgitant volume (RVol) method.
Doppler
- Late systolic peak
- ![[Mitral Valve Prolapse MVP-1745638089042.webp]]
Malignant MVP
![[Mitral Valve Prolapse MVP-1745637451858.webp]] Figure source Transthoracic echocardiography demonstrating myxomatous bileaflet mitral valve prolapse (arrows) in cases 1 (A), 2 (B), and 3 (C). High-velocity mid-systolic spike (lateral annulus, 32 cm/s) in cases 1 (D), 2 (mid-systole, 19 cm/s) (E), and 3 (late systole, 25 cm/s) (F). (G) Normal medial annulus systolic velocity, case 1. (H) Tugging of the posteromedial papillary muscle by prolapsing leaflets (arrow), case 4. (I) Late-peaking systolic tissue velocity spike of 24 cm/s, case 4. (J) Pickelhaube, spiked German military helmet (reprinted with permission from the collection of Peter Suciu).
Management
- Severe MR
- quantitative Doppler assessments are recommended to define severe MR more precisely, e.g. a regurgitant volume of at least 60 mL, a regurgitant fraction of at least 50%, and an effective regurgitant orifice of at least 40 mm.1
- Patients who have severe mitral regurgitation with symptoms or with left ventricular dysfunction (ejection fraction, <60%), dilatation (left ventricular end-systolic dimension, >40 mm), or both should be offered surgery.
- Asymptomatic patients without left ventricular dysfunction or dilatation but with atrial fibrillation or pulmonary hypertension should be considered for surgery.
- There are two options for surgical correction of severe mitral regurgitation due to mitral-valve prolapse: valve replacement or valve repair.
Mitral valve replacement
Mitral-valve replacement can be performed with the use of either a mechanical or a biologic prosthesis. However, there are several drawbacks to mitral-valve replacement. These include the need for lifelong anticoagulation therapy and the risk of thromboembolism with the use of mechanical valves; the risk of prosthetic-valve deterioration and failure with the use of bioprosthetic valves; and the risk of prosthetic-valve endocarditis. In addition, if the chordae tendineae are severed during surgery, the ventricular wall is no longer anchored to the valve apparatus, and the tethering effect of the chordae is lost. As a result, left ventricular wall stress increases and left ventricular function deteriorates.
Mitral valve repair
- The goals of mitral-valve repair are to obtain a proper line of coaptation on both leaflets, to correct annular dilatation, and to preserve (or repair, if necessary) the subvalvular apparatus.
- In patients with isolated prolapse of the posterior middle scallop (P2), which is encountered in the majority of patients with degenerative mitral regurgitation, repair usually involves limited resection of this scallop, including the removal of the minimum number possible of adjacent chordae and supporting apparatus. The remaining segments of the posterior leaflet, namely P1 and P3, are then brought together.
- Annuloplasty ring
- The annulus, which is distorted or dilated or both, is stabilized with an annuloplasty ring or band.
- Repairs of the anterior leaflet, either in isolation or with concomitant posterior leaflet repair, are more complex procedures that are best handled by surgeons who are experienced in mitral repair.
-1762813105433.webp) Figure source: 1
Figure source: 1
Footnotes
-
Verma S, Mesana TG. Mitral-valve repair for mitral-valve prolapse. N Engl J Med. 2009 Dec 3;361(23):2261-9. doi: 10.1056/NEJMct0806111. PMID: 19955526. ↩ ↩2 ↩3 ↩4 ↩5 ↩6 ↩7 ↩8 ↩9
-
Morningstar, J. E., Nieman, A., Wang, C., Beck, T., Harvey, A., & Norris, R. A. (2021). Mitral Valve Prolapse and Its Motley Crew‐Syndromic Prevalence, Pathophysiology, and Progression of a Common Heart Condition. Journal of the American Heart Association, 10(13). https://doi.org/10.1161/jaha.121.020919 ↩ ↩2 ↩3