# Mitral valve prolapse
Any VAs complicating MVP should not be considered idiopathic or benign
- History of CTD (often myxomatous MVP), unexplained syncope
- Risk is higher when they present with unexplained syncope or even presyncope than when they present with isolated palpitations and lower when asymptomatic
- Family history of MVP, SCD, arrhythmias, cardiomyopathy
- 12-lead ECG
- Look for risk factors for arrhythmogenesis: TWI inferior and/or lateral, QRS fractionation, prolonged QTc, polymorphic ventricular ectopy, NSVT
- Event monitor for arrhythmia severity classification and prognostication (even if ASx)
- Mild VA (PVC >= 5% and/or VT runs <120 bpm), Moderate VA (VT runs 120-179 bpm), Severe VA (VT >= 180 and/or hx of sustained VT/VF)
- Further investigation if multifocal ventricular ectopy, short coupled PVCs (coupling interval <350 ms) or fast NSVT as they are at risk of SCD
- Echocardiogram to evaluate for SHD, MR, LV systolic dysfunction, *bileaflet prolapse*, severe myxomatous degeneration with redundant leaflets, MAD
- Consider Cardiac MRI to evaluate for evidence of MAD, LGE
- AMVP post-cardiac arrest, sustained VA (prior to secondary prev ICD), unexplained syncope, NSVT, phenotypic risk feature (palpitations, TWI, polymorphic PVCs, MAD, redundant MV leaflets, enlarged LA, EF <=50%)
- Consider Genetic testing, cascade screening
- Consider ILR
- if unexplained syncope with unrevealing event monitor, AMVP, high risk features (sustained VT, NSVT, unexplained syncope), LGE on CMR
- Consider exercise stress testing to assess suspected exercise-induced VAs
- Consider EP referral for ICD
- Consider BB, CCB if symptomatic from PVCs
- Flecainide, Propafenone and Amiodarone yield more potent PVC burden reduction with frequent improvement in LV function. Sotalol can reduce the PVC burden effectively but failed to improve LV function.- Mitral-valve prolapse is defined as the displacement of some portion of one or both leaflets of the mitral valve into the left atrium during systole.1
- Mitral valve prolapse (MVP) is the most common valvular heart disease (prevalence of 2% to 3% across the general population) and historically was considered benign in the absence of severe MR and normal left ventricular function.2
- The natural history of mitral-valve prolapse is heterogeneous and is largely determined by the severity of mitral regurgitation. Although a majority of patients remain asymptomatic and may have a near-normal life expectancy, ==approximately 5 to 10% have progression to severe MR==.1
- Involves myxomatous degeneration of the mitral valve leaflets → eventually leads to structural incompetence and superior displacement of one or both mitral leaflets into the left atrium (LA) during systole 3
- 2 main etiologies:2
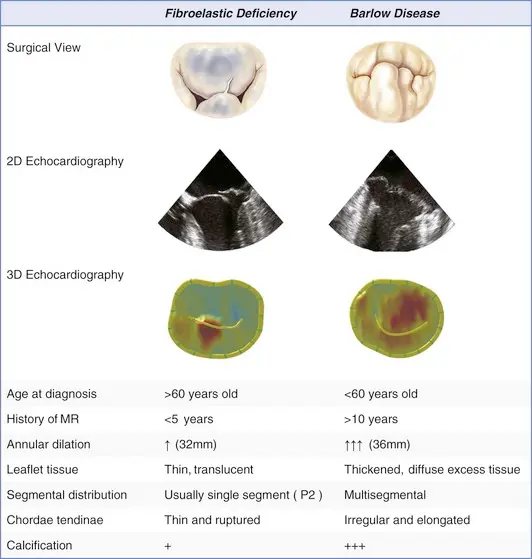
- Myxomatous MVP (Barlow’s disease)
- Characterized by excess tissue including chordal thickening/elongation, annular dilation and calcification with low probability of chordal rupture.
- Fibroelastic deficiency
- Characterized by chordal thinning, elongation, and/or high probability of rupture, with classic findings of prolapse and MR of varying severity. This is the most common form of MVP.
- Myxomatous MVP (Barlow’s disease)
- Genetics
- Parental MVP was associated with a ∼5 fold increase in the risk of MVP, and first degree relatives have a 30–50% likelihood of being affected.
- Taken as a whole, long term survival of subjects with MVP is not different from the general population, with its prognosis determined mainly by MR severity.
- Association with connective tissue diseases
- The incidence of MVP in Marfan syndrome patients is linked with age, with up to 75% affected at >60 years.
- The reported prevalence of MVP is up to 45% in patients with Loeys-Dietz syndrome.
- The prevalence of MVP is approximately 6% in patients with Ehlers-Danlos syndrome.
- Patients with osteogenesis imperfecta and adult polycystic kidney disease are also frequently reported to have MVP.
- Ambulatory ECG Monitoring
- Recently, large cohorts of consecutive patients with isolated MVP, comprehensively characterized with 24-hour-Holter-monitoring, clinical and echocardiographic assessment, demonstrated that VAs are frequent but are rarely severe.
- Severe arrhythmias are independently linked to excess mortality accumulating over time, irrespective of the severity of degenerative MR. Thus it appears that there is a phenotype of MVP prone to developing VAs over time with increased risk of mortality and SCD when VAs become severe.
- Left untreated, mitral valve prolapse with severe MR results in limiting symptoms, left ventricular dysfunction, HF, pulmonary hypertension, and AFib.1
- ⚠️ Patients who experience an increased burden of premature ectopic beats (such as PVCs) are at an increased risk of developing more severe, even life‐threatening arrhythmias.3
- There is a <1% risk of SCD in MVP. Key risk factors include severe associated MR and left ventricular (LV) systolic dysfunction.
- MAD results in an abnormal motion of the mitral annulus, termed “curling”. It is associated with increased risk of arrhythmias and is therefore and integral component of the arrhythmic MVP (AMVP) complex.2
-1763405474526.webp) Figure source: 2
Figure source: 2
Anatomy
- Mitral-valve prolapse is characterized predominantly by myxomatous degeneration.1
- In younger patients, the disease is often manifested by excess leaflet tissue and is known as Barlow’s syndrome, the most extreme form of myxomatous degeneration.
- On the other hand, in older patients 👵, the prolapsing mitral valve tends not to have excess leaflet tissue, an entity known as fibroelastic deficiency.
- Both conditions can lead to leaflet prolapse and chordal elongation or rupture, representing the spectrum of degenerative mitral-valve disease.
- Involves myxomatous degeneration of the mitral valve leaflets → eventually leads to structural incompetence and superior displacement of one or both mitral leaflets into the left atrium (LA) during systole 3
- Anatomic abnormalities result in the mitral orifice not closing completely during systole, causing regurgitation.1
- Annular dilatation may also develop over time, leading to further progression of mitral regurgitation.1
Barlow disease
- diffuse, myxoid degeneration of the MV → excess tissue in multiple valve segments, including leaflets and chordae → (typically) both anterior and posterior MVP, as well as annular dilation
- Compared to fibroelastic deficiency, Barlow disease is less frequently associated with chordal rupture.
Fibroelastic deficiency
- Caused by abnormalities in connective tissue → leaflet redundancy, chordal thinning and elongation → MVP
- The thinned and elongated tendinous chords in MVP are prone to rupture
- Chordal rupture may lead to flail leaflet and a sudden ↑ in MR volume (acute MR)
Malignant MVP
![[Mitral Valve Prolapse MVP-1745637451858.webp]] Figure source Transthoracic echocardiography demonstrating myxomatous bileaflet mitral valve prolapse (arrows) in cases 1 (A), 2 (B), and 3 (C). High-velocity mid-systolic spike (lateral annulus, 32 cm/s) in cases 1 (D), 2 (mid-systole, 19 cm/s) (E), and 3 (late systole, 25 cm/s) (F). (G) Normal medial annulus systolic velocity, case 1. (H) Tugging of the posteromedial papillary muscle by prolapsing leaflets (arrow), case 4. (I) Late-peaking systolic tissue velocity spike of 24 cm/s, case 4. (J) Pickelhaube, spiked German military helmet (reprinted with permission from the collection of Peter Suciu).
Arrhythmic mitral valve prolapse (A-MVP) syndrome
- Clinically challenging to identify those at risk of lethal arrhythmias who may benefit from a primary prevention ICD. 4
- These patients have a greater risk of complex arrhythmias and SCD in the presence of normal LV function and nonsevere MR.4
- A-MVP syndrome is clinically defined by the presence of MVP with evidence of frequent or complex ventricular arrhythmias, in the absence of an alternative well-defined arrhythmic substrate.4
-1763408622549.webp) Figure source: 4
Figure source: 4
Diagnosis
- Physical Exam
- 🩺: mid‐systolic “click” heard best at the apex
- presence of a late systolic or holosystolic murmur can suggest the presence of MR
- 🩺: mid‐systolic “click” heard best at the apex
- Diagnosis
- Echo (gold standard)
- TTE to assess the mechanism and severity of mitral regurgitation, as well as LA, LV size and function
- ECG to assess cardiac rhythm
- Echo (gold standard)
ECG
- Electrophysiological markers of A-MVP syndrome include T-wave inversion in inferior and lateral leads, QTc prolongation, and fragmented QRS complexes. Complex/frequent ventricular arrhythmias, including polymorphic ventricular ectopy (PVE) from papillary muscles and fascicular regions and nonsustained ventricular tachycardia (NSVT), are also linked.4
- Fragmented QRS (fQRS)
-1763406599270.webp) Figure source: 2. Twelve-lead electrocardiogram (ECG) showing T wave inversion in infero-lateral leads. An ECG of a 44-year-old female who presented with ventricular fibrillation and was found to have mitral valve prolapse. The tracing is remarkable for T wave inversion in leads II, III, AVF, V4-V6.
Figure source: 2. Twelve-lead electrocardiogram (ECG) showing T wave inversion in infero-lateral leads. An ECG of a 44-year-old female who presented with ventricular fibrillation and was found to have mitral valve prolapse. The tracing is remarkable for T wave inversion in leads II, III, AVF, V4-V6.
Echo
- Using echocardiography, MVP is diagnosed ideally in the PLAX window as systolic displacement of the mitral leaflet into the LA of ≥2 mm from the mitral annular plane.
- If parasternal windows are of poor quality, the apical long-axis view can also be used, although the latter is less standardized and thus more variable.
- ⛔ Diagnosis of MVP should be avoided in the A4C or A2C windows
-1763405505399.webp)
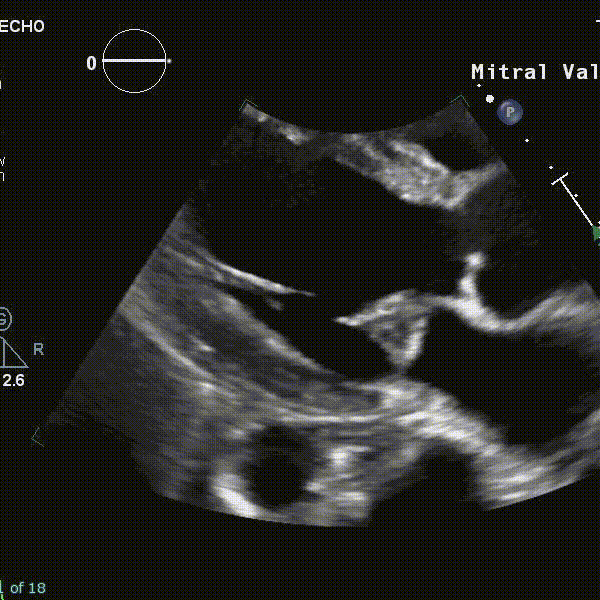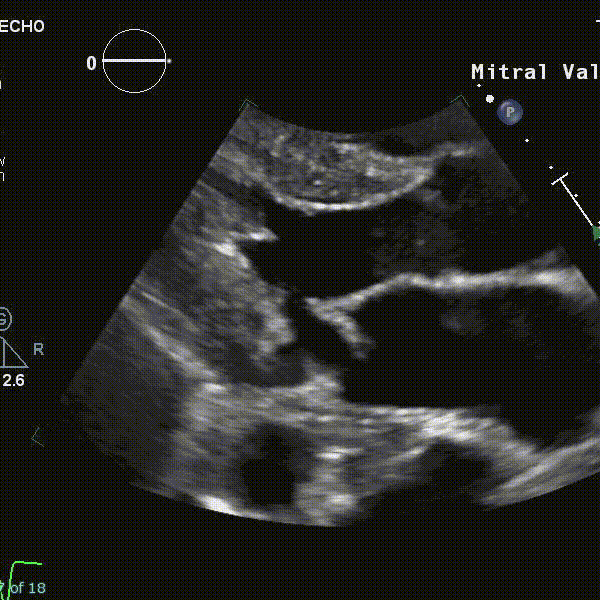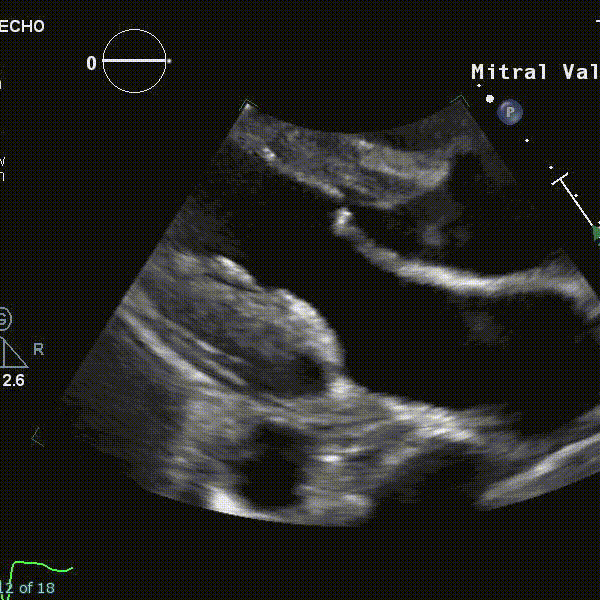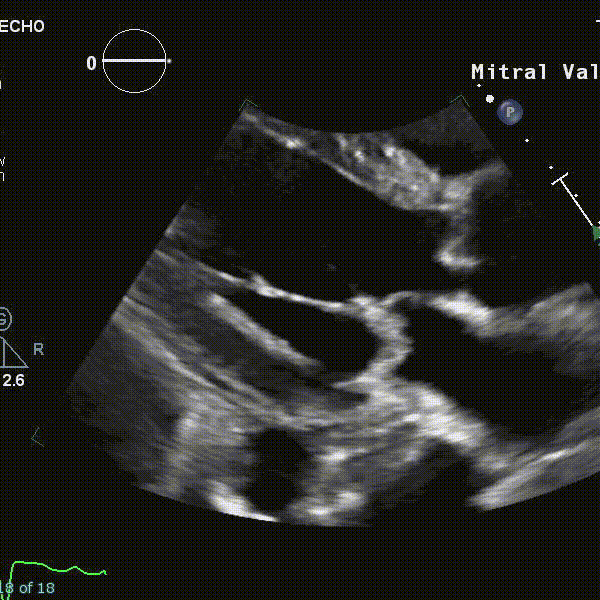
- Structural abnormalities that increased risk of A-MVP syndrome include increasing degrees of myxomatous degeneration of valve leaflets, leaflet redundancy, bi-leaflet prolapse, and the presence of mitral annular disjunction (MAD)
MR quantification in MVP
⛔ Avoid using single frame measurements - PISA EROA and VCW - when the MR is not holosystolic, e.g. MVP with late systolic MR, functional MR with biphasic MR, LV dyssynchrony with early systolic MR, as they can overestimate MR severity.
When non-holosystolic, you should use regurgitant volume (RVol) method.
Doppler
- Late systolic peak
- ![[Mitral Valve Prolapse MVP-1745638089042.webp]]
Cardiac MRI
- LGE: suggestive of myocardial scar, located specifically around the mitral apparatus and adjacent LV wall, has also been associated
Management
- Severe MR
- quantitative Doppler assessments are recommended to define severe MR more precisely, e.g. a regurgitant volume of at least 60 mL, a regurgitant fraction of at least 50%, and an effective regurgitant orifice of at least 40 mm.1
- Patients who have severe mitral regurgitation with symptoms or with left ventricular dysfunction (ejection fraction, <60%), dilatation (left ventricular end-systolic dimension, >40 mm), or both should be offered surgery.
- Asymptomatic patients without left ventricular dysfunction or dilatation but with atrial fibrillation or pulmonary hypertension should be considered for surgery.
- There are two options for surgical correction of severe mitral regurgitation due to mitral-valve prolapse: valve replacement or valve repair.
Surgery
- Although mitral-valve prolapse is more common in women, more men are referred for surgery.1
-1762813105433.webp) Figure source: 1
Figure source: 1
Mitral valve replacement
Mitral-valve replacement can be performed with the use of either a mechanical or a biologic prosthesis. However, there are several drawbacks to mitral-valve replacement. These include the need for lifelong anticoagulation therapy and the risk of thromboembolism with the use of mechanical valves; the risk of prosthetic-valve deterioration and failure with the use of bioprosthetic valves; and the risk of prosthetic-valve endocarditis. In addition, if the chordae tendineae are severed during surgery, the ventricular wall is no longer anchored to the valve apparatus, and the tethering effect of the chordae is lost. As a result, left ventricular wall stress increases and left ventricular function deteriorates.
Mitral valve repair
- The goals of mitral-valve repair are to obtain a proper line of coaptation on both leaflets, to correct annular dilatation, and to preserve (or repair, if necessary) the subvalvular apparatus.
- In patients with isolated prolapse of the posterior middle scallop (P2), which is encountered in the majority of patients with degenerative mitral regurgitation, repair usually involves limited resection of this scallop, including the removal of the minimum number possible of adjacent chordae and supporting apparatus. The remaining segments of the posterior leaflet, namely P1 and P3, are then brought together.
- Annuloplasty ring
- The annulus, which is distorted or dilated or both, is stabilized with an annuloplasty ring or band.
- Repairs of the anterior leaflet, either in isolation or with concomitant posterior leaflet repair, are more complex procedures that are best handled by surgeons who are experienced in mitral repair.
Footnotes
-
Verma S, Mesana TG. Mitral-valve repair for mitral-valve prolapse. N Engl J Med. 2009 Dec 3;361(23):2261-9. doi: 10.1056/NEJMct0806111. PMID: 19955526. ↩ ↩2 ↩3 ↩4 ↩5 ↩6 ↩7 ↩8
-
Sabbag A, Essayagh B, Barrera JDR, Basso C, Berni A, Cosyns B, Deharo JC, Deneke T, Di Biase L, Enriquez-Sarano M, Donal E, Imai K, Lim HS, Marsan NA, Turagam MK, Peichl P, Po SS, Haugaa KH, Shah D, de Riva Silva M, Bertrand P, Saba M, Dweck M, Townsend SN, Ngarmukos T, Fenelon G, Santangeli P, Sade LE, Corrado D, Lambiase P, Sanders P, Delacrétaz E, Jahangir A, Kaufman ES, Saggu DK, Pierard L, Delgado V, Lancellotti P. EHRA expert consensus statement on arrhythmic mitral valve prolapse and mitral annular disjunction complex in collaboration with the ESC Council on valvular heart disease and the European Association of Cardiovascular Imaging endorsed cby the Heart Rhythm Society, by the Asia Pacific Heart Rhythm Society, and by the Latin American Heart Rhythm Society. Europace. 2022 Dec 9;24(12):1981-2003. doi: 10.1093/europace/euac125. PMID: 35951656; PMCID: PMC11636573. ↩ ↩2 ↩3 ↩4 ↩5 ↩6 ↩7
-
Morningstar, J. E., Nieman, A., Wang, C., Beck, T., Harvey, A., & Norris, R. A. (2021). Mitral Valve Prolapse and Its Motley Crew‐Syndromic Prevalence, Pathophysiology, and Progression of a Common Heart Condition. Journal of the American Heart Association, 10(13). https://doi.org/10.1161/jaha.121.020919 ↩ ↩2 ↩3
-
Ghelani R, Chow JJ, Miyazawa A, Artico J, Cole G, Kanagaratnam P, Peters NS, Varnava A. The Arrhythmic Mitral Valve Prolapse Syndrome: A New Insight for Understanding the Arrhythmogenic Substrate? JACC Case Rep. 2025 May 28;30(12):103419. doi: 10.1016/j.jaccas.2025.103419. Epub 2025 Apr 24. PMID: 40447364; PMCID: PMC12235458. ↩ ↩2 ↩3 ↩4 ↩5